A general term for fats and oils. Lipids include triglycerides (simple fats), phospholipids (important constituents of cell membranes and nerve tissue), and sterols, such as cholesterol.
or
lipid is a general term for naturally occuring fats found in the body.
There are many different types of lipids including cholesterol and triglycerides.
HDL (good cholesterol) and LDL (bad cholesterol) are both lipids, as are saturated, unsaturated, and trans-fats.
Lipids are involved in many body functions including the synthesis of new cells and the production of many hormones.
lipids: Fats
Fats are composed of three fatty acids and glycerol. These triglycerides can be solid or liquid at room temperature. Those that are solid are classified as fats, while those that are liquid are known as oils. Fatty acids consist of a long chain of carbons with a carboxyl group at one end. Depending on their structure, fatty acids can be saturated or unsaturated. While fats have been denigrated to the point that many believe that fat should be eliminated from the diet, fat serves many useful purposes. Fats store energy, help to insulate the body and cushion and protect organs.Lipids: Phospholipids
A phospholipid is composed of two fatty acids, a glycerol unit, a phosphate group and a polar molecule. The phosphate group and polar head region of the molecule is hydrophillic (attracted to water), while the fatty acid tail is hydrophobic (repelled by water). When placed in water, phospholipids will orient themselves into a bilayer in which the nonpolar tail region faces the inner area of the bilayer. The polar head region faces outward and interacts with the water. Phospholipids are a major component of cell membranes which enclose the cytoplasm and other contents of a cell.Lipids: Steroids and Waxes
Steroids have a carbon backbone that consists of four fused ring-like structures. Steroids include cholesterol, sex hormones (progesterone, estrogen and testosterone) and cortisone. Waxes are comprised of an ester of a long-chain alcohol and a fatty acid. Many plants have leaves and fruits with wax coatings to help prevent water loss. Some animals also have wax-coated fur or feathers to repel water. Unlike most waxes, ear wax is composed of phospholipids and esters of cholesterol.Bilayer sheet
The preferred structure of lipids in aqueous solutions are usually a bilayer sheet of lipids rather than spherical micelles. This is because the two fatty acid chains are too big and bulky to fit into the interior of a micelle. Therefore, micelles usually have one hydrocarbon chain instead of two. Lipid bilayers" form rapidly and spontaneously in an aqueous media and are stabilized by hydrophobic interactions, Van der Waals attractive forces, electrostatic interactions and hydrogen-bonding. The function of the lipid bilayer is to form a barrier between the two sides of the membrane. Due to the fact that the lipid bilayer consists of hydrophobic fatty acid chains, ions and most polar molecules have trouble passing through the bilayer. The one exception to this rule is water because water has a high concentration, small size, and a lack of a complete charge. In order for a molecule to pass through the lipid bilayer it must move from an aqueous environment to a hydrophobic environment and then back into an aqueous environment.Therefore the permeability of small molecules is related to the solubility of said molecule in a nonpolar solvent versus the solubility of the molecule in water.
Micelles can also have a structure that is inside out of its normal structure. Instead of having the hydrocarbon chains inside, they can face outside and while the polar heads are arranged inside the sphere. This happens in a "water in oil" situation because there is so much oil surrounding the drop of water that the hydrocarbon chains face outside instead of inside.

Size
The size of a micelle is usually 200 A or 20 nm. The size of a micelle is more limited than that of a lipid bilayer. A lipid bilayer can span up to 107 A or 106 nm.
The lipid bilayer is not a rigid structures, rather they are quite fluid. The individual lipid molecules are able to move or diffuse laterally across the membrane quite easily, this process is called lateral diffusion. However, lipids have much more trouble flipping from one side of the membrane to the other ,this process is called traverse diffusion or flip, because this would involve the polar head traveling through the hydrophobic core, and this interaction between polar and hydrophobic regions is unfavorable. So the lipid can move around laterally at a rate of about 2 micrometers per second, while it takes a much longer amount of time to flip flop.
the fluidity of a lipid bilayer also depends on both the temperature and the hydrocarbon chain. As the temperature is increased the fluidity of the lipid bilayer increases as well. Also the more cis double bonds the hydrocarbon tail has the more fluid the structure becomes. This is because when the hydrocarbon tail has cis double bonds it can no longer pack as well as the saturated hydrocarbon tail, so it becomes more fluid. Also the longer the hydrocarbon tail, the higher the transition temperature, which is the temperature at which the bilayer goes from rigid to fluid, this is because longer hydrocarbon tails can interact more strongly than shorter chains.
Formation
Micelles form when the polar head and the non polar tails arrange in a special way. They are usually driven to arrange either with the polar heads out (oil in water) or with the polar head in (water in oil). Micelles only form when the concentration of surfactant is greater than the critical micelle concentration (CMC). The surfactant is any surface active material that can part the surface upon entering. The CMC is the concentration above surfactant when micelles will form spontaneously. The higher the concentration, the more micelles there are. Micelle formation also depend on the Krafft temperature. This temperature is when surfactants will form micelles. If the temperature if below the Krafft temperature, then there is no spontaneous formation of micelles. As the temperature increases, the surfactant will turn into a soluble form and be able to form micelles from a crystalline state. The hydrophobic effect is also a driving force that needs to be taken into account. This effect is characterized by the fact that like to form intermolecular aggregates in aqueous substances and in intramolecular molecules. Micelle formation can be summed up by thermodynamics, driven by entropy and enthalpy.Function and Usage
Micelles usually form in soap molecules. Soap often form as micelles because they contain only one hydrocarbon chain instead of two. Therefore they make up the soap property. Micelles act as emulsifiers that allows a compound that is usually insoluble in water to dissolve. Detergents and soap work by insert the long hydrophobic tails from soap into the insoluble dirt (such as oil) while the hydrophilic head face outside and surround the nonpolar dirt. Then, this micelle can be washed away since the outside of the micelle is soluble with the solvent, which is usually polar. This is the reason why soap helps clean oily and waxy substances off from dishes since water alone cannot pull the oil off.
Micelles are also at work in the human body. Micelles help the body absorb lipid and fat soluble vitamins. They help the small intestine to absorb essential lipids and vitamins from the liver and gall bladder. They also carry complex lipids such as lecithin and lipid soluble vitamins (A, D, E and K) to the small intestine. Without micelles, these vitamins will not be able to be absorbed into the body which will lead to serious complications. Micelles also help clean the skin. Many facial washes use micelles to perform this task. They clean the skin by removing oil and other substances without the need of being washed afterward.
Vesicles

The picture above shows how liposomes are formed. The vesicles trap the glycine after sonication. Sonication disperses the phospholipids into equal size vesicles of about 500 A or 50 nm diameter sizes. The phospholipids form vesicles around the many molecules of glycine floating around. This is driven by the hydrophobic forces. After gel filtration, the vesicles are then separated from the rest of the glycine floating around. The function of this can be transport or storage of glycine to the appropriate targets. An enlarged view shows the single strand micelles around the hydrophobic glycine. The tails are inside with the glycine because they are hydrophobic while the heads face the outside which is surrounded by water.
All Lipids are hydrophobic: that’s the one property they have in common. This group of molecules includes fats and oils, waxes, phospholipids, steroids (like cholesterol), and some other related compounds.
![[Glycerol]](http://biology.clc.uc.edu/graphics/bio104/glycerol.jpg) | Fats and oils are made from two kinds of molecules: glycerol (a type of alcohol with a hydroxyl group on each of its three carbons) and three fatty acids joined by dehydration synthesis. Since there are three fatty acids attached, these are known as triglycerides. “Bread” and pastries from a “bread factory” often contain mono- and diglycerides as “dough conditioners.” Can you figure out what these molecules would look like? The main distinction between fats and oils is whether they’re solid or liquid at room temperature, and this, as we’ll soon see, is based on differences in the structures of the fatty acids they contain. |
|---|
Structure of Fatty Acids
![[Fatty Acids]](http://biology.clc.uc.edu/graphics/bio104/fatty%20acid.jpg) The terms saturated, mono-unsaturated, and poly-unsaturated refer to the number of hydrogens attached to the hydrocarbon tails of the fatty acids as compared to the number of double bonds between carbon atoms in the tail. Fats, which are mostly from animal sources, have all single bonds between the carbons in their fatty acid tails, thus all the carbons are also bonded to the maximum number of hydrogens possible. Since the fatty acids in these triglycerides contain the maximum possible amouunt of hydrogens, these would be called saturated fats. The hydrocarbon chains in these fatty acids are, thus, fairly straight and can pack closely together, making these fats solid at room temperature. Oils, mostly from plant sources, have some double bonds between some of the carbons in the hydrocarbon tail, causing bends or “kinks” in the shape of the molecules. Because some of the carbons share double bonds, they’re not bonded to as many hydrogens as they could if they weren’t double bonded to each other. Therefore these oils are called unsaturated fats. Because of the kinks in the hydrocarbon tails, unsaturated fats can’t pack as closely together, making them liquid at room temperature. Many people have heard that the unsaturated fats are “healthier” than the saturated ones. Hydrogenated vegetable oil (as in shortening and commercial peanut butters where a solid consistency is sought) started out as “good” unsaturated oil. However, this commercial product has had all the double bonds artificially broken and hydrogens artificially added (in a chemistry lab-type setting) to turn it into saturated fat that bears no resemblance to the original oil from which it came (so it will be solid at room temperature).
The terms saturated, mono-unsaturated, and poly-unsaturated refer to the number of hydrogens attached to the hydrocarbon tails of the fatty acids as compared to the number of double bonds between carbon atoms in the tail. Fats, which are mostly from animal sources, have all single bonds between the carbons in their fatty acid tails, thus all the carbons are also bonded to the maximum number of hydrogens possible. Since the fatty acids in these triglycerides contain the maximum possible amouunt of hydrogens, these would be called saturated fats. The hydrocarbon chains in these fatty acids are, thus, fairly straight and can pack closely together, making these fats solid at room temperature. Oils, mostly from plant sources, have some double bonds between some of the carbons in the hydrocarbon tail, causing bends or “kinks” in the shape of the molecules. Because some of the carbons share double bonds, they’re not bonded to as many hydrogens as they could if they weren’t double bonded to each other. Therefore these oils are called unsaturated fats. Because of the kinks in the hydrocarbon tails, unsaturated fats can’t pack as closely together, making them liquid at room temperature. Many people have heard that the unsaturated fats are “healthier” than the saturated ones. Hydrogenated vegetable oil (as in shortening and commercial peanut butters where a solid consistency is sought) started out as “good” unsaturated oil. However, this commercial product has had all the double bonds artificially broken and hydrogens artificially added (in a chemistry lab-type setting) to turn it into saturated fat that bears no resemblance to the original oil from which it came (so it will be solid at room temperature).![[Cis and Trans Bonds]](http://biology.clc.uc.edu/graphics/bio104/cistrans.jpg) In unsaturated fatty acids, there are two ways the pieces of the hydrocarbon tail can be arranged around a C=C double bond. In cis bonds, the two pieces of the carbon chain on either side of the double bond are either both “up” or both “down,” such that both are on the same side of the molecule. In trans bonds, the two pieces of the molecule are on opposite sides of the double bond, that is, one “up” and one “down” across from each other. Naturally-occurring unsaturated vegetable oils have almost all cis bonds, but using oil for frying causes some of the cis bonds to convert to trans bonds. If oil is used only once like when you fry an egg, only a few of the bonds do this so it’s not too bad. However, if oil is constantly reused, like in fast food French fry machines, more and more of the cis bonds are changed to trans until significant numbers of fatty acids with trans bonds build up. The reason this is of concern is that fatty acids with trans bonds are carcinogenic, or cancer-causing. The levels of trans fatty acids in highly-processed, lipid-containing products such as margarine are quite high, and I have heard that the government is considering requiring that the amounts of trans fatty acids in such products be listed on the labels.
In unsaturated fatty acids, there are two ways the pieces of the hydrocarbon tail can be arranged around a C=C double bond. In cis bonds, the two pieces of the carbon chain on either side of the double bond are either both “up” or both “down,” such that both are on the same side of the molecule. In trans bonds, the two pieces of the molecule are on opposite sides of the double bond, that is, one “up” and one “down” across from each other. Naturally-occurring unsaturated vegetable oils have almost all cis bonds, but using oil for frying causes some of the cis bonds to convert to trans bonds. If oil is used only once like when you fry an egg, only a few of the bonds do this so it’s not too bad. However, if oil is constantly reused, like in fast food French fry machines, more and more of the cis bonds are changed to trans until significant numbers of fatty acids with trans bonds build up. The reason this is of concern is that fatty acids with trans bonds are carcinogenic, or cancer-causing. The levels of trans fatty acids in highly-processed, lipid-containing products such as margarine are quite high, and I have heard that the government is considering requiring that the amounts of trans fatty acids in such products be listed on the labels. We need fats in our bodies and in our diet. Animals in general use fat for energy storage because fat stores 9 KCal/g of energy. Plants, which don’t move around, can afford to store food for energy in a less compact but more easily accessible form, so they use starch (a carbohydrate, NOT A LIPID) for energy storage. Carbohydrates and proteins store only 4 KCal/g of energy, so fat stores over twice as much energy/gram as carbohydrates or proteins. By the way, this is also related to the idea behind some of the high-carbohydrate weight loss diets. The human body burns carbohydrates and fats for fuel in a given proportion to each other. The theory behind these diets is that if they supply carbohydrates but not fats, then it is hoped that the fat needed to balance with the sugar will be taken from the dieter’s body stores. Fat is also is used in our bodies to a) cushion vital organs like the kidneys and b) serve as insulation, especially just beneath the skin.
Phospholipids
![[Lecithin]](http://biology.clc.uc.edu/graphics/bio104/lecithin.jpg) Phospholipids are made from glycerol, two fatty acids, and (in place of the third fatty acid) a phosphate group with some other molecule attached to its other end. The hydrocarbon tails of the fatty acids are still hydrophobic, but the phosphate group end of the molecule is hydrophilic because of the oxygens with all of their pairs of unshared electrons. This means that phospholipids are soluble in both water and oil.
Phospholipids are made from glycerol, two fatty acids, and (in place of the third fatty acid) a phosphate group with some other molecule attached to its other end. The hydrocarbon tails of the fatty acids are still hydrophobic, but the phosphate group end of the molecule is hydrophilic because of the oxygens with all of their pairs of unshared electrons. This means that phospholipids are soluble in both water and oil.An emulsifying agent is a substance which is soluble in both oil and water, thus enabling the two to mix. A “famous” phospholipid is lecithin which is found in egg yolk and soybeans. Egg yolk is mostly water but has a lot of lipids, especially cholesterol, which are needed by the developing chick. Lecithin is used to emulsify the lipids and hold them in the water as an emulsion. Lecithin is the basis of the classic emulsion known as mayonnaise.
![[Phospholipid Bilayer]](http://biology.clc.uc.edu/graphics/bio104/membrane.jpg) Our cell membranes are made mostly of phospholipids arranged in a double layer with the tails from both layers “inside” (facing toward each other) and the heads facing “out” (toward the watery environment) on both surfaces.
Our cell membranes are made mostly of phospholipids arranged in a double layer with the tails from both layers “inside” (facing toward each other) and the heads facing “out” (toward the watery environment) on both surfaces.
Steroids
The general structure of cholesterol consists of two six-membered rings side-by-side and sharing one side in common, a third six-membered ring off the top corner of the right ring, and a five-membered ring attached to the right side of that. The central core of this molecule, consisting of four fused rings, is shared by all steroids, including estrogen (estradiol), progesterone, corticosteroids such as cortisol (cortisone), aldosterone, testosterone, and Vitamin D. In the various types of steroids, various other groups/molecules are attached around the edges. Know how to draw the four rings that make up the central structure.Cholesterol is not a “bad guy!” Our bodies make about 2 g of cholesterol per day, and that makes up about 85% of blood cholesterol, while only about 15% comes from dietary sources. Cholesterol is the precursor to our sex hormones and Vitamin D. Vitamin D is formed by the action of UV light in sunlight on cholesterol molecules that have “risen” to near the surface of the skin. At least one source I read suggested that people not shower immediately after being in the sun, but wait at least ½ hour for the new Vitamin D to be absorbed deeper into the skin. Our cell membranes contain a lot of cholesterol (in between the phospholipids) to help keep them “fluid” even when our cells are exposed to cooler temperatures.
Many people have hear the claims that egg yolk contains too much cholesterol, thus should not be eaten. An interesting study was done at Purdue University a number of years ago to test this. Men in one group each ate an egg a day, while men in another group were not allowed to eat eggs. Each of these groups was further subdivided such that half the men got “lots” of exercise while the other half were “couch potatoes.” The results of this experiment showed no significant difference in blood cholesterol levels between egg-eaters and non-egg-eaters while there was a very significant difference between the men who got exercise and those who didn’t.
BIOLOGICAL IMPORTANCE OF LIPIDS:
ENERGY SOURCE: Lipids act as fuel in the body. Lipids are found to be superior to carbohydrates and protein in providing energy to body with respect to their energy released by their oxidation. Lipids yield 9.5Kcal/gram whereas proteins and carbohydrates yield only about 4Kcal/gram.
LIPID STORAGE: Since lipids are insoluble in aqueous solution, they can be stored easily in body. They can be stored in the body in almost unlimited amount in contrast to carbohydrates which is stored in limited amount and places.
INSULATION: Fats are usually stored in the subcutaneous layers. They exert insulating property. This is found to be useful especially useful in those animals living in the cold climate like whale etc., Lipids stored around internal organs found to be padding and protecting the organs.
STRUCTURAL ROLE: Lipids play a vital role in structure by being the important component of lipid bilayer in membrane. Especially phospholipids and cholesterols are important.
ENDOCRINE FUNCTION: Hormones like adrenocorticoids, mineralocorticoids, sex hormones and vitamin D were found to be synthesized from lipids like cholesterol.
VITAMIN ABSORPTION: Lipids are essential for the absorption of fat soluble vitamins like vitamin A, D, E and K.
NERVOUS SYSTEM: Lipids are found to be important constituent of the nervous system because nerve cells found to contain greater amount of lipids they play vital role nerve cell insulation and impulse conduction.
ANTIBIOTIC ACTIVITY: Squalamine, a steroid from Sharks' blood, has been found to be antifungal and antibacterial activity.
CLASSIFICATION OF LIPIDS:
There is no single, internationally accepted system of classification for the lipids available. The names of these compounds, however, do fall into certain categories as the component structures present are considered. Bloor's classification is generally adopted with a few modifications as follows:
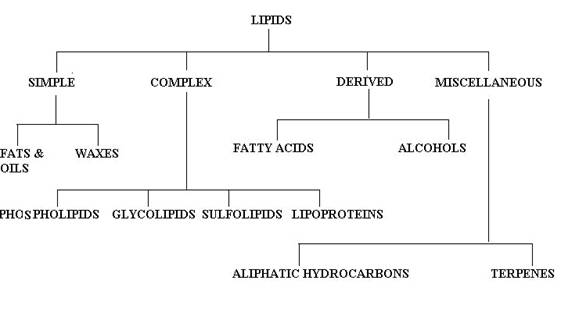
SIMPLE LIPIDS:
Simple lipids are esters of fatty acids with various alcohols. They contain mainly fatty acids and alcohols alone. They are further divided into two classes namely, Neutral fats and waxes.
Neutral fats:
They are triesters of fatty acids with glycerol. Triacyglycerol is an example for Neutral fats.
waxes:
Waxes are esters of fatty acids with higher mono hydroxy aliphatic alcohols. True waxes, cholesterol esters and vitamin A and D esters are example for waxes.
COMPOUND LIPIDS:
They are the esters of fatty acids containing groups, other than and in addition, to an alcohol and fatty acids.
a) PHOSPHOLIPIDS:
In addition to fatty acids and alcohol presence, they also contain phosphorous, nitrogenous bases and other substitution groups. Lecithin and cephalins are examples for phospholipids.
b) GLYCOLIPIDS:
Lipids containing carbohydrates are referred as glycolipids. They contain an special alcohol moiety called sphingosine or sphingol and nitrogenous base. They do not have phosphorous. Gangliosides and cerebrosides are examples of compounds lipids.
c) SULPHOLIPIDS:
Lipids with sulfate groups are referred as sulpholipids.
d) LIPOPROTEINS:
When lipids contain protein then they are known as lipoproteins. Example chylomicrons, VLDL, LDL and HDL.
DERIVED LIPIDS:
Derived lipids are lipids obtained upon hydrolysis of the simple and complex lipids and still retaining the characteristics of lipids. They are classified further into two types namely fattyacid and alcohol.
a) FATTY ACID:
They are the hydrolyzed products of simple and complex lipids. They mainly of mono carboxylic acids. They may be saturated or unsaturated. Their length varies between C4 to C30. Palmitic Acid C16
b) ALCOHOL:
It includes molecules with OH group as functional group. It also varies from simple straight chain alcohol like glycerol to complex cyclic alcohols like cholesterol.
MISCELLANEOUS:
This includes the lipids which can not be grouped under any of the above headings. They include
* Aliphatic hydrocarbons include iso-octadecane found in liver fat and certain hydrocarbons found in bees wax and plant waxes.
* Terpenes
* Carotenoids.
* Squalene is a hydrocarbon found in shark and mammalian liver and human sebum.
* Vitamin E and K.
PROPERTIES OF FATS:
Properties of fats and oils depend upon the fatty acids and alcohol which are present in it. Properties of fats and oils are studied under two headings namely Physical and Chemical properties.
PHYSICAL PROPERTIES:
1. State:
Fats containing saturated fatty acids are solid at room temperature. The animal fats usually solid due to saturated fatty acids. Most plant fats, in contrast, possess unsaturated fatty acids and are, henceforth, liquid at room temperature.
2. Color, odour and taste:
When pure, the fats are colorless, virtually odorless and possess an extremely bland taste. They are capable of absorbing a variety of odors and hence flavor during storage. For example, the perfumes of some flowers can be isolated by placing their petals in contact with the fat for a certain period, then extracting the fat with alcohol and concentrating the essence.
3. Solubility:
The fats are only sparingly soluble in water. These are, therefore, described as hydrophobic in contrast to the water soluble substances like proteins. However, these are freely soluble in organic solvents like chloroform, ether, acetone and benzene. These solvents, as they dissolve fats in them, are also known as fat solvents. The solubility of the fatty acids in organic solvents decreases with the increase of chain length. The introduction of hydroxyl groups, however, increases solubility.
4. Melting Point:
The melting point of fats depends on the chain length and the degree of unsaturation. Melting point increases with increase in their chain length but increase in the degree of unsaturation lowers melting point.
5. Specific gravity:
The specific gravity of the fats is less than 1 i.e. 0.86. Therefore, they float on water surface. Solid fats are lighter than the liquid fats.
6. Geometric Isomerism:
Presence of double bonds in unsaturated fatty acids is responsible for the geometric or cis-trans isomerism.
7. Insulation:
The fats possess high insulating power, i.e., they are bad conductor of heat. A layer of fat below the skin provides a sort of blanket for warm-blooded animals or homoiotherms. This is especially important for whales and seals which have to maintain a high temperature in cold wastes. The fishes are cold-blooded animals or poikiltherms and therefore, do not require maintenance of high temperature and so have very little subcutaneous fat.
8. Emulsification:
It is the process by which a lipid mass is converted into a number of small lipid droplets. The fats may be emulsified by shaking either with water or with emulsifying agents like soaps, gums, proteins, etc. An emulsifying agent helps in the production of a finely divided suspension of a fat in an aqueous medium. The hydrocarbon portions of the two tend to aggregate. This leaves the water-soluble group of the emulsifier projecting into the aqueous phase. A fat droplet will associate with a number of molecules of the emulsifier, thus producing a new water-soluble surface. Water molecules, henceforth, tend to be held in a layer or cloud around each droplet, thus disallowing the aggregation of the fat droplets. The process of emulsification is of great metabolic significance. In fact, the fats have to be emulsified before they can be absorbed by the intestinal wall. The process is accomplished by the bile juice secreted from liver.
9. Surface tension:
When liquid fat is poured on water, it spread uniformly over the surface of water in the form of a unimolecule layer and thus reduces the surface tension of water.HEMICAL PROPERTIES:
Chemical properties of fats studied under three different headings namely
1. Reactions due to COOH group
2. Reactions due to Double bonds
3. Reactions due to OH group
1. REACTIONS DUE TO –COOH GROUP:
A. Hydrolysis by Enzyme:
The fats are hydrolyzed by the enzymes lipases to yield fatty acids and glycerol. This reaction occurs in three steps at a slightly alkaline pH. The fats first split to produce diglycerides, part of these are then split to monoglycerides. Finally, part of the monoglycerides split to yield to glycerol and fatty acid. This reaction plays significant role in digestion of lipids. In the intestine, the absorption of mono, di and tri glycerides is rapid, so that very little free glycerol is formed during fats digestion.
B. Hydrolysis by Alkali (Saponification):
The hydrolysis of fats by alkali is called as saponification. This reaction results in the formation of glycerol and salts of fatty acids which are called as soaps. The soaps are of two types namely hard and soft. Hard soaps such as the common bar soaps are the sodium salts of the higher fatty acids. Soft soaps are the potassium salts of higher fatty acids and are marketed as semisolids or pastes. The fatty acid salts of calcium, magnesium, zinc and lead are, however, insoluble in water. Calcium soaps are used industrially as lubricating greases. Zinc soaps are employed in the manufacture of talcum powder and other cosmetics. Lead and magnesium soaps are used in paints industry to hasten the process drying.
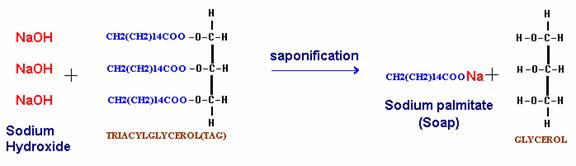
Soaps are important cleansing agents. Their cleansing property is due to their emulsifying action i.e. capacity to render more prolonged the mixing of oil and water. This is accomplished by means of negative charge the soap anion confers on oil droplets. The electrostatic repulsion then prevents the coalescence of soap and oil droplets into an oil phase.
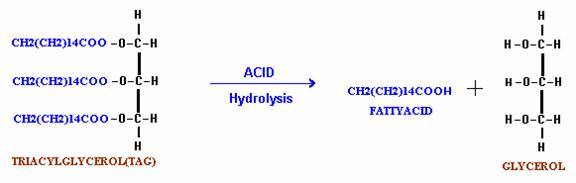
C. Hydrolysis by Acids:
When fats are hydrolyzed by acid, it yields only fatty acids and glycerol.
D. Hydrolysis by Microbes:
When butter or other fats are stored, they often become rancid and hence unpalatable. Rancidity is caused by the growth of microorganisms which secrete enzymes like lipases. These split the fats into glycerol and free fatty acids. The fatty acids impart unpleasant odour and flavor to the fat. Since, rancidity aroused due to the hydrolysis reaction, it is known as hydrolytic rancidity. However, butter may be prevented from becoming rancid by refrigeration or by exclusion of water.
QUANTITATIVE TESTS:
The chemical reactions give valuable information about the chemical nature of fatty acids and the number of hydroxyl groups present in the fat molecule. Such chemical determinations involve various analytical tests. These are called chemical constants. They are used to identify a pure fat, assess the degree of adulteration and determine the proportions of different types of fat in a mixture. The constants used for these tests are include Saponification Number, Acid Number. Polenske Number, Reichert-Meissl Number, Iodine number and Acetyl Number.
1. Saponification Number:
The number of milligrams of KOH required to saponify the free and combined fatty acids in one gram of a given fat is called as its saponification number. The amount of alkali needed to saponify a given quantity of fat will depend upon the number of carboxylic group present. Thus fats containing short chain fatty acids will have more carboxylic groups per gram than long chain fatty acids and this will take up more alkali and hence will have higher saponification number. Butter containing a larger proportion of short chain fatty acids, such as butyric and caproic acids, has relatively high saponification number from 220 to 230. Oleo-margarine, with more long chain fatty acids, has saponification number of 195 or less.
2. Acid Number:
Number of milligrams of KOH required to neutralize the fatty acids in one gram of fat is known as the acid number. The acid number indicates the degree of rancidity of a given fat. The acid number, thus, determine the quantity of free fatty acid present in a fat. The fat which has been both processed and stored properly has a very low acid number.
3. Polenske Number:
The number of millilitre of 0.1N KOH required to neutralize the insoluble fatty acids which are those not volatile with steam distillation, present in 5 gram of fat is referred as Polenske number.
4. Reicher-Meissl Number:
It is the number of milliliters of 0.1N KOH required to neutralize the soluble volatile fatty acids which are distilled from 5 gram of fat. The Reichert-Meissl measures the amount of volatile soluble fatty acids. By saponification of fat, acidification and steam distillation, the volatile soluble acids may be separated and determined quantitatively. Butter fat is the only common fat with a high RM number and this determination, therefore, is of interest in that it aids the food chemist in detecting butter substitutes in food products.
5. Iodine Number:
Iodine Number is defined as the number of grams of iodine absorbed by 100 gram of fat. Iodine number is a measure of the degree of unsaturation of a fat. The more the iodine number, the greater the degree of unsaturation. The determination of iodine number is useful to the chemist in determining the quality of oil or its freedom from adulteration. Iodine number of cotton seed oil varies from 103 to 111 that of olive oil from 79 to 88, and that of linseed oil from 175 to 202. A commercial lot of olive oil which has iodine number higher than 88 might have been adulterated with cotton seed oil. Again a batch of linseed oil with iodine number lower than 175 might also have been adulterated with the cotton seed oil.
6. Acetyl Number:
The number of milligrams of KOH required to neutralize the acetic acid released by saponification of one gram of fat after it has been acetylated is known as acetyl number. Some of the fatty acid residues in fats contain hydroxyl groups. In order to determine the proportion of these, they are acetylated by means of acetic anhydride. Thus an acetyl group is introduced wherever a free –OH group is present. After washing out the excess acetic anhydride and acetic acid liberated, the acetylated fat can be dried and weighed and the acetic acid in combination determined by titration with standard alkali after it has been set free. The acetyl number is thus a measure of the number of hydroxyl group present.
Castor oil because of its high content of ricinoleic acid has a high acetyl number. Caster oil 146-150 and Olive oil 10.5. Acetyl number can be used to detect adulteration.
Lipid Blood Tests
Total Cholesterol (TC)
Directly linked to risk of heart and blood vessel disease.Goal values:
- 75-169 mg/dL for those age 20 and younger
- 100-199 mg/dL for those over age 21
Preparation:
This test may be measured any time of the day without fasting. However, if the test is drawn as part of a total lipid profile, it requires a 12-hour fast (no food or drink, except water). For the most accurate results, wait at least two months after a heart attack, surgery, infection, injury or pregnancy to check cholesterol levels.Cholesterol is a type of fat, found in your blood. It is produced by your body and also comes from the foods you eat (animal products). Cholesterol is needed by your body to maintain the health of your cells. Too much cholesterol leads to coronary artery disease. Your blood cholesterol level is related to the foods you eat or to genetic conditions (passed down from other generations of family members).
High Density Lipoprotein (HDL) “Good cholesterol”
High levels linked to a reduced risk of heart and blood vessel disease. The higher your HDL level, the better.Goal value:
- Greater than 40 mg/dL
Preparation:
This test may be measured any time of the day without fasting. However, if the test is drawn as part of a total lipid profile, it requires a 12-hour fast (no food or drink, except water). For the most accurate results, wait at least two months after a heart attack, surgery, infection, injury or pregnancy to check HDL levels.HDL is a lipoprotein (a combination of fat and protein) found in the blood. It is called "good" cholesterol because it removes excess cholesterol from the blood and takes it to the liver. A high HDL level is related to lower risk of heart and blood vessel disease.
Low Density Lipoprotein (LDL) “Bad cholesterol”
High levels are linked to an increased risk of heart and blood vessel disease, inlcuding coronary artery disease, heart attack and death. Reducing LDL levels is a major treatment target for cholesterol-lowering medications.Goal values:
- Less than 70 mg/dL for those with heart or blood vessel disease and for other patients at very high risk of heart disease (those with metabolic syndrome)
- Less than 100 mg/dL for high risk patients (e.g., some patients who have multiple heart disease risk factors)
- Less than 130 mg/dL for individuals who are at low risk for coronary artery disease
Preparation:
Blood should be collected after a 12-hour fast (no food or drink, except water). For the most accurate results, wait at least 2 months after a heart attack, surgery, infection, injury or pregnancy to check LDL levels.LDL is a lipoprotein (a combination of fat and protein) found in the blood. It is called "bad" cholesterol because it picks up cholesterol from the blood and takes it to the cells. A high LDL level is related to a higher risk of heart and blood vessel disease.
Triglycerides (TG)
Elevated in obese or diabetic patients. Level increases from eating simple sugars or drinking alcohol. Associated with heart and blood vessel disease.Goal value:
- Less than 150 mg/dl
Preparation:
Blood should be collected after a 12-hour fast (no food or drink, except water). For the most accurate results, wait at least 2 months after a heart attack, surgery, infection, injury or pregnancy to check triglyceride levels.Triglycerides are a type of fat found in the blood. The blood level of this type of fat is most affected by the foods you eat (such as sugar, fat or alcohol) but can also be high due to being overweight, having thyroid or liver disease and genetic conditions. High levels of triglycerides are related to a higher risk of heart and blood vessel disease.

No comments:
Post a Comment